|
 |
| J Rhinol > Volume 29(2); 2022 |
|
Abstract
Endoscopic skull-base surgery (ESBS) is a rapidly growing surgical area that involves collaboration of otolaryngology-head and neck surgeons and neurosurgeons. Various tumor pathologies and extents have been successfully treated with ESBS, and diverse reconstruction methods have been adopted since its introduction. The optimal reconstructive strategy should be based on heterogeneous surgical situations and tumor extent. Nevertheless, there are few current guidelines for selecting reconstructive methods. Therefore, we review diverse options for endoscopic skull-base reconstruction.
Endoscopic endonasal skull base surgery has been popularized and expanded over the past two decades. Endoscopic pituitary surgery via a transsphenoidal approach is the basic technique, and expanded approaches (such as the endoscopic transplanum, transclival, or transcribriform approaches) have gained popularity as regular surgical techniques. Endoscopic endonasal approaches (EEAs) have many advantages over more ŌĆ£openŌĆØ transcranial approaches, including better visualization and improved cosmesis for the patient as well as not requiring brain retraction or manipulation.
However, EEA was initially criticized for its possible higher rate of postoperative cerebrospinal fluid (CSF) leaks, especially when used to remove large intradural lesions such as meningiomas, craniopharyngiomas, skull-base invading malignancies, and clival chordomas through large skull-base dural defects. Although introduction of the nasoseptal flap (NSF) method (also known as the Hadad-Bassagasteguy flap) has reduced the rate of postoperative CSF leaks, reconstruction is challenging and remains one of the most important steps in endoscopic skull-base surgery (ESBS). In this article, we review the surgical technique and describe operative nuances of endoscopic reconstruction of graded CSF leaks after endoscopic endonasal skull-base surgeries.
The goal of reconstruction is to achieve a water-tight closure between the intracranial space and sinonasal cavity to prevent CSF leakage, pneumocephalus, and meningitis. In a recent meta-analysis [1,2] that assessed risk factors for post-operative CSF leakage, obesity, perioperative radiotherapy, and high intraoperative CSF flow rate affected CSF leakage after EEA. In Korean populations, few people are severely obese (BMI>30 kg/m2); therefore, obesity is not a common risk factor for Korean patients who undergo EEA. In regular clinical situations, the grade of intraoperative CSF leakage and the sites of CSF leakage were the most important factors to select the methods for reconstruction. As previously described by Esposito et al. [3], grading is categorized as follows: grade 0, no observed leak; grade I, a small ŌĆ£weepingŌĆØ CSF leak confirmed by the Valsalva maneuver without a visible diaphragmatic defect; grade II, a moderate leak with a definite diaphragmatic defect; or grade III, a large diaphragmatic and/or dural defect created as part of a suprasellar planum or transclival extended transsphenoidal approach. Because KellyŌĆÖs grading system was developed based on a transsellar approach, the location of the defect should be considered when planning a successful reconstruction. A posterior fossa defect that arises after a transclival approach has been considered an independent higher risk factor of CSF leakage compared with an anterior defect repaired using a transcribriform approach or a suprasellar defect treated with a transplanum approach [4]. For both low-flow and high-flow CSF leaks, use of a multilayer closure method has shown higher success rates than single-layer reconstruction. Therefore, the most cost-effective reconstruction that carries the lowest morbidity should be selected for individual cases. According to the recently published International Consensus Statement [5], a vascular flap is recommended for large dural defects and high-flow CSF leaks, while a free graft is recommended for low-flow CSF leaks.
Although the NSF is the most effective method for grade I to III leaks, a NSF can negatively impact the patientŌĆÖs sinonasal quality of life and olfactory function [6,7]. Therefore, NSF should only be used when absolutely necessary, such as in grade II or grade III leaks in patients with other risk factors.
Despite the reconstruction method, the main purpose and basic principles of skull-base reconstruction are as follows: 1) creating a protective barrier to reduce the chance of infection, 2) reduction of the dead space left by the tumor resection, and 3) prevention of the descent of the chiasm and other intracranial contents.
In the past, most textbooks have introduced the method of sellar packing with autologous fat, muscle, and fascia following a transsphenoidal approach for pituitary tumors and have made the rigid support using bone or cartilage as well. However, various methods of reconstruction of the skull-base have been introduced over the last 20 years, and many studies have reported that not using sellar packing does not increase the incidence rates of postoperative CSF leaks or other complications [8]. In addition, overpacking can cause compression of the optic chiasm, and caution is required around the optic chiasm during suprasellar reconstruction.
Various materials (including homologous or autologous grafts) have been used for endoscopic skull-base reconstruction. These materials vary depending on the grade and site of the CSF leaks, but they all used for a multilayer reconstruction using an underlay (subdural) and an overlay (epidural and under the skull-base bone) (Table 1).
Autografts from the free mucosa, fat, and fascia lata have the advantage of being both safe and cost-effective. Autologous fat has a strong advantage in that it can fill in the dead space left in the subdural space after tumor resection. The fascia lata affords a layered, water-tight reconstruction. However, there are also disadvantages of using these materials: additional operation time is required, an additional incision is needed at the donor site, and there are limitations in designing the size or shape of the grafts.
Another option is a free mucosal graft, which can be obtained simply from intranasal structures (the septum and inferior or middle turbinate) and has the advantage of not causing additional donor complications after surgery. A turbinate can be used as the material for an onlay graft by stripping away the mucosa after excision of the structure. In this case, if the mucous membrane of the border is not completely flattened, a mucocele can occur later; care must be taken to ensure that it is sufficiently compressed and spread out.
A free bone graft is also useful because it can provide strong support against intracranial pressure and can be obtained from the septum (the vomer or perpendicular plate of the ethmoid bone) during posterior septectomy. However, the use of bone tissue in patients requiring scheduled radiation therapy must be approached with caution because it can cause osteoradionecrosis or graft breakdown [9,10].
Allografts do not require additional time or a donor site incision to obtain materials, but they are associated with additional costs and have disadvantages in that they can be used only after careful evaluation of biocompatibility. Acellular dermal grafts, such as Alloderm® grafts (LifeCell Corp., The Woodlands, TX, USA), have been effectively used for a long time [11]; a recent study reported blood vessels regenerated into an Alloderm® graft used for skull-base reconstruction, proving its effectiveness in the reconstruction of the skull base [12]. In Korea, Megaderm® (L&C Bio, Seoul, Korea) is one alternative to Alloderm® that has a lower cost.
Tensor fascia lata donated from cadavers have been used in some institutions, and their effectiveness and safety are established [13]. In our experience, there was no difference between autologous grafts and allografts in terms of postoperative CSF leaks or meningitis (unpublished data).
The pedicled NSF is a workhorse flap that uses the septal branch of the sphenopalatine artery as a pedicle. It can be harvested without an additional incision and can be used even for large skull-base defects because it has sufficient length and width and also offers the advantage of being able to rotate freely. After the introduction of these flap techniques in 2006, it was reported that CSF leaks could be reduced to less than 5% [14]. In addition, even when patients require postoperative radiation therapy, post-treatment complications are minimized due to the abundant blood supply present in these flaps. Although the NSF is a robust flap used for endoscopic skullbase reconstruction, a septal flap can reduce the patientŌĆÖs quality of life, including alterations in olfaction. Therefore, the NSF should be used selectively only when absolutely necessary for reconstruction in case of high-flow CSF leaks that arise after some pituitary adenoma, tuberculum sellae meningioma, craniopharyngioma, and clival chordoma surgery.
Inferior or middle turbinate flap with or without the lateral nasal wall could also be an alternative when an NSF is unavailable due to previous usage or tumor invasion. However, these types of flaps are technically difficult to harvest, and vascularity is not as good as that seen in NSFs. In addition, rotation of the flap is limited. Therefore, a turbinate flap could be used for parasellar or clival defects in limited situations.
Anteiror ethmoidal artery-based flap could be used for anterior cranial fossa or posterior table of frontal sinus. Both anterior based septal flap or anterior based lateral nasal wall flap are good alternative options instead of posterior based NSF.
Although a pedicled NSF and other intranasal flaps are the reconstructive option of choice for most endoscopic skullbase defects, different vascularized flaps might be necessary in some cases to achieve optimal outcomes. A pericranial flap, temporoparietal fascial flap, or a palatal flap are extranasal options. All of these alternative vascularized flaps are preferred to avascular grafting options because the rate of CSF leaks is lower with a vascularized repair.
If other reconstructive methods are unavailable or unsuccessful, a free flap is the last option. It is difficult to perform an endoscopic approach alone, and precise microvascular anastomosis is required in these cases. In particular, it is useful when there is persistent necrosis due to a large amount of radiation in the central skull base and when reconstruction has failed several times [15].
Whichever reconstruction method is chosen, absorbable materials (such as fibrin-coated collagen fleece [Tachosil┬«, Nycomed, Linz, Austria] or fibrin glue) play an important supporting role in multilayer reconstruction. Each material is chosen according to the experience of the surgeon. Several studies [16,17] have shown the effectiveness of Tachosil┬« for the reconstruction of grade IŌĆōII CSF leaks. However, the effectiveness of fibrin glue is not yet clear [18].
Although a diverse grading system and reconstruction methods [16,19] are used, we introduce our preferred methods using KellyŌĆÖs grading system as follows:
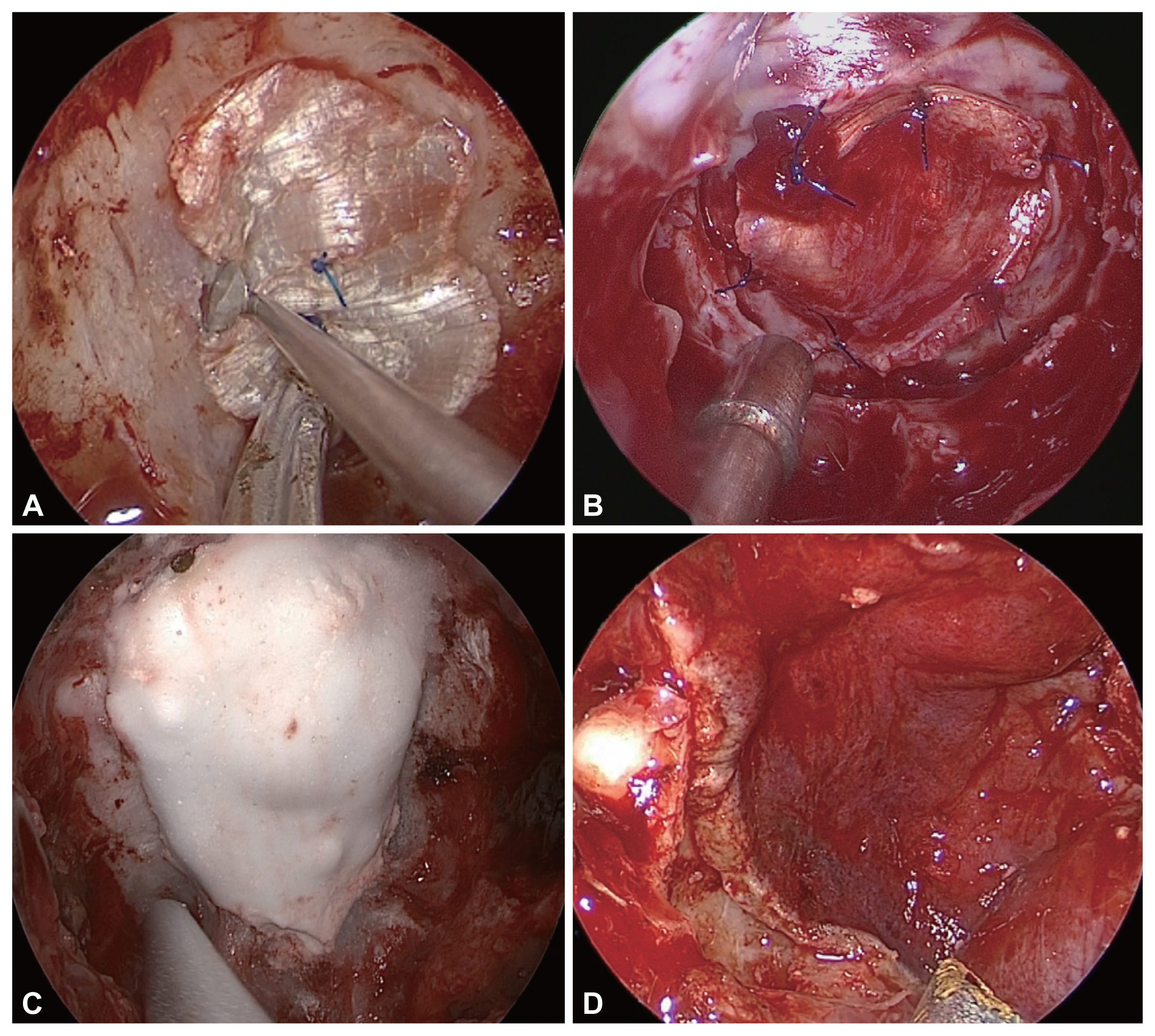
If there is no CSF leakage during pituitary surgery, it is classified as grade 0. However, even in this case, since the arachnoid membrane is very thin (Fig. 1A) or CSF leakage that was not detected during the operation can occur after the operation, proper reconstruction is necessary. In this case, depending on the surgeonŌĆÖs choice, an autologous mucosal graft [20] or sphenoid mucosal flap [21] is typically considered. When approaching the sphenoid sinus, the surgeon creates a superior or inferior based sphenoid mucosal flap and then resects the tumor. After tumor resection, Tachosil┬« (collagen fleece coated with a fibrin sealant) is inserted intradurally, and the sphenoid mucosal flap is repositioned on the sellar floor (Fig. 1B). If the arachnoid is bulging out to the sellar floor, the surgeon could add a bony reconstruction using a sphenoid bone flap or septal bone graft. After that, mucosal flap is covered over the sella and the fibrin glue is applied. This method can reduce the postoperative CSF leakage and preserves as much of the normal mucosal membrane as possible and maintains the mucociliary transport and aeration of the sphenoid sinus.
The same technique is used as for grade 0, and a Tachosil® patch is placed at the exact site of the tear in the arachnoid membrane. Next, the surgeon should confirm water-tight sealing using a Valsalva maneuver. CSF leakage caused by the space between the anterior dural edge and the normal gland can be safely reconstructed by dural clipping (Fig. 2) [22]. In this situation, a lumbar drain is not used.
If there is a clear CSF leak intraoperatively and there is a definite arachnoid defect, Tachosil® patch is placed on the arachnoid hole and the dead space is filled with autologous fat grafting, and a mucosal flap or nasal mucosa free graft is performed. If possible, rigid buttress such as septal bone could give more consistent outcome. In this case as well, a lumbar drain is not placed.
High-pressure CSF leaks usually occur in cases of giant pituitary adenomas, tuberculum sellae/olfactory groove meningiomas, craniopharyngiomas, or clival chordomas. A multilayer reconstruction with an NSF is the preferred approach to address grade III leakage.
After tumor removal and meticulous hemostasis, it is important to strip the skull-base mucosa (especially in the sphenoid sinus) to allow flap adherence to the bone. The septa of the sphenoid sinus or the ethmoid cells must be flattened to prevent dead space between the skull-base bone and the NSF.
The NSF should be harvested at the beginning of the surgery and rotated it into the nasopharynx during the main procedure. A monopolar cautery is used to make the incision along the nasal floor, which enables a fast flap design without bleeding. We used a No. 15 blade knife and iris scissors to make the incisions for anterior and superior flap designs to protect the septal cartilage and preserve the olfactory neuroepithelium. In terms of flap design, a long and wide flap is preferred. The anterior incision should be made 1 cm posterior to the caudal septum to prevent saddle nose when exposed cartilage is necrotized. In addition, the inferior incision should be made laterally along the mucoperiosteum of the nasal floor. To ensure adequate coverage of the skull-base defect, it is better to overestimate the defect size and harvest a larger flap than to have a smaller flap with suboptimal coverage.
Meticulous multilayer reconstruction is critical to prevent postoperative CSF leakage. Defects accompanied by high-flow leaks must be converted to low-flow states by placing a piece of autologous or homologous fascia lata or an acellular dermal graft. We prefer a button type inlay-onlay graft with an acellular dermal graft or homologous fascia lata (Fig. 3A). This suture prevents the inlay graft from displacing into the intracranial space. If a more water-tight reconstruction is desired, a direct dural suture with a free graft could be used (Fig. 3B). In cases with posterior fossa defects, we prefer large autologous fat grafts to prevent brainstem herniation. Recently, we have used injectable hydroxyapatite between onlay graft and NSF (Fig. 3C) because it provides more rigid reconstruction and more consistent results. When we use the hydroxyapatite, it must be covered totally by vascularized flap after maximal bleeding control to avoid crack. If not, chronic sinonasal or intracranial infection could happen. However, there is longer term evidence using hydroxyapatite and we must know possible long-term adverse effects.
Before positioning the NSF, it is important to aggressively remove the rostrum and sphenoid floor to prevent a hinge in the flap. Care must be taken to maintain proper orientation of the flap so that the mucoperichondrial/mucoperiosteal surface of the NSF is in direct contact with the skull-base defect. It is also important to keep the vascular pedicle from twisting. Using a four-hand technique, the NSF is carefully positioned over the defect. Gentle pressure is then applied to the flap in a proximal to distal fashion to prevent dead space between the flap and skull base (Fig. 3D).
Definite indications of lumbar drainage after endoscopic skull-base reconstruction have not been determined. A previous meta-analysis [2] and systemic review [23] demonstrated that lumbar drains do not reduce the incidence of postoperative CSF leaks. However, because individual surgeons tend to insert lumber drains when the reconstruction is not as solid as desired, the systemic review of retrospective studies could not show a high level of evidence for their use.
A Pittsburgh group [24] published a first prospective randomized controlled trial on the effectiveness of lumbar drainage after ESBS. They showed a significant reduction in CSF leakage in patients that had lumbar drains placed for anterior and posterior skull-base defects. They also reported a tendency for less CSF leakage in the lumbar drainage group with suprasellar pathology. These findings suggest that endoscopic skull-base reconstruction is not a perfect method, and adjuvant techniques (such as rigid reconstruction or lumbar drainage) could improve its success rate [25,26].
It remains controversial whether sellar floor reconstruction should be performed in the absence of intraoperative CSF leakage. Although the approach will depend on the propensity of the surgeon, reconstruction using an appropriate synthetic graft material and mucosal graft/flap is thought to be one of the best options. Various reconstruction strategies can be used based on the operatorŌĆÖs experience and surgical findings. In cases of grade II or higher CSF leakage, multi-layer reconstruction using autologous fat grafts or pedicled NSFs is essential. As with safe and complete tumor resection, the establishment of various reconstruction strategies for different situations is a key factor in determining the success of ESBS.
Notes
Fig.┬Ā1
Sellar floor reconstruction for grade 0 or I cerebrospinal fluid leaks. A: A bulging thinned arachnoid membrane following tumor resection. The white arrow indicates the arachnoid membrane. B: A superior based sphenoid mucosal flap was used to cover the sellar defect.

Fig.┬Ā2
Clipping for CSF leak between dura and normal gland. A: A grade I CSF leak from the anterior edge between the dura and normal gland (white arrow). B: Dural clipping on the anterior dural edge. CSF, cerebrospinal fluid.
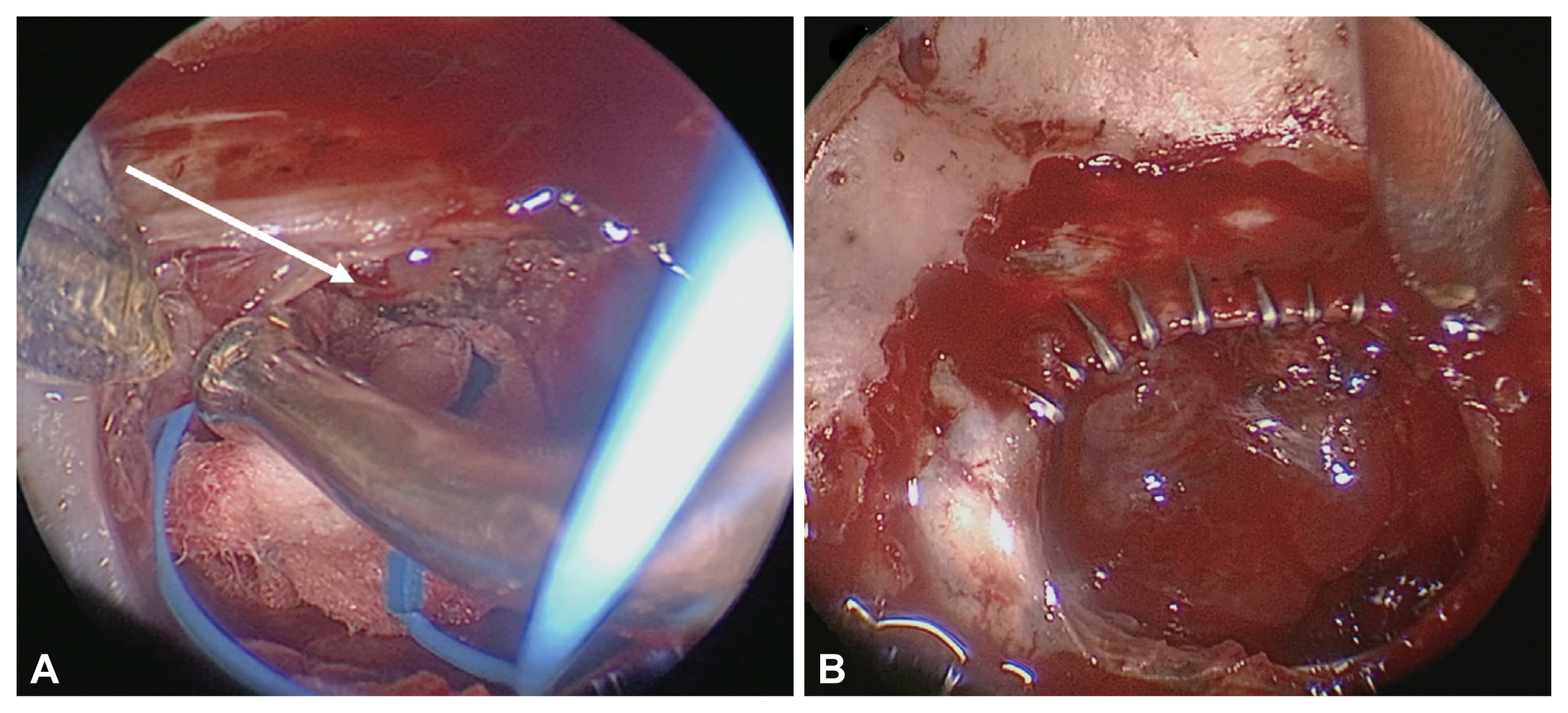
Fig.┬Ā3
Reconstruction options for grade III cerebrospinal fluid leakage. A: An inlay and onlay fascia graft with a button-type suture. B: A direct dura-fascia suture to ensure a water-tight reconstruction. C: A rigid reconstruction using hydroxyapatite. D: A nasoseptal flap reconstruction after multilayer graft (A, B, and/or C).
Table┬Ā1
Methods of endoscopic skull-base reconstruction
REFERENCES
1) Kim JS, Hong SD. Risk factors for postoperative CSF leakage after endonasal endoscopic skull base surgery: a meta-analysis and systematic review. Rhinology 2021;59(1):10ŌĆō20.


2) Ahmed OH, Marcus S, Tauber JR, Wang B, Fang Y, Lebowitz RA. Efficacy of perioperative lumbar drainage following endonasal endoscopic cerebrospinal fluid leak repair: a meta-analysis. Otolaryngol Head Neck Surg 2017;156(1):52ŌĆō60.


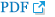
3) Esposito F, Dusick JR, Fatemi N, Kelly DF. Graded repair of cranial base defects and cerebrospinal fluid leaks in transsphenoidal surgery. Oper Neurosurg (Hagerstown) 2007 60(4 Suppl 2):295ŌĆō303. discussion 303ŌĆō4.


4) Fraser S, Gardner PA, Koutourousiou M, Kubik M, Fernandez-Miranda JC, Snyderman CH, et al. Risk factors associated with postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery. J Neurosurg 2018;128(4):1066ŌĆō71.


5) Wang EW, Gardner PA, Zanation AM. International consensus statement on endoscopic skull-base surgery: executive summary. Int Forum Allergy Rhinol 2019;9(S3):S127ŌĆō44.


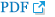
6) Seo MY, Nam DH, Kong DS, Lee SH, Noh Y, Jung YG, et al. Extended approach or usage of nasoseptal flap is a risk factor for olfactory dysfunction after endoscopic anterior skullbase surgery: results from 928 patients in a single tertiary center. Rhinology 2020;58(6):574ŌĆō80.


7) Seo MY, Nam DH, Kong DS, Lee JJ, Ryu G, Kim HY, et al. Quality of life after extended versus transsellar endoscopic skull base surgery from 767 patients. Laryngoscope 2019;129(6):1318ŌĆō24.


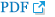
8) Seda L, Camara RB, Cukiert A, Burattini JA, Mariani PP. Sellar floor reconstruction after transsphenoidal surgery using fibrin glue without grafting or implants: technical note. Surg Neurol 2006 66(1):46ŌĆō9. discussion 49.


9) Clavenna MJ, Turner JH, Chandra RK. Pedicled flaps in endoscopic skull base reconstruction: review of current techniques. Curr Opin Otolaryngol Head Neck Surg 2015;23(1):71ŌĆō7.

10) Zuniga MG, Turner JH, Chandra RK. Updates in anterior skull base reconstruction. Curr Opin Otolaryngol Head Neck Surg 2016;24(1):75ŌĆō82.


11) Germani RM, Vivero R, Herzallah IR, Casiano RR. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am J Rhinol 2007;21(5):615ŌĆō8.


12) Taufique ZM, Bhatt N, Zagzag D, Lebowitz RA, Lieberman SM. Revascularization of AlloDerm used during endoscopic skull base surgery. J Neurol Surg B Skull Base 2019;80(1):46ŌĆō50.



13) Kim S, Jeon C, Kong DS, Park K, Kim JH. Clinical efficacy of radiation-sterilized allografts for sellar reconstruction after transsphenoidal surgery. J Korean Neurosurg Soc 2011;50(6):503ŌĆō6.



14) Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 2006;116(10):1882ŌĆō6.


15) Sigler AC, D'Anza B, Lobo BC, Woodard TD, Recinos PF, Sindwani R. Endoscopic skull base reconstruction: an evolution of materials and methods. Otolaryngol Clin North Am 2017;50(3):643ŌĆō53.

16) Cho JM, Ahn JY, Chang JH, Kim SH. Prevention of cerebrospinal fluid rhinorrhea after transsphenoidal surgery by collagen fleece coated with fibrin sealant without autologous tissue graft or postoperative lumbar drainage. Neurosurgery 2011 68(1 Suppl Operative):130ŌĆō6. discussion 136ŌĆō7.


17) So J, Park H, Sung KS, Lee KS, Hong CK. Sandwich technique using fibrin-coated collagen fleece for sellar reconstruction in large dural defects during transsphenoidal surgery. J Clin Neurosci 2017;43:256ŌĆō60.


18) Ganesh PB, Basavarajaiah BM, Rudrappa BA, Kasaragod SK. Cerebrospinal fluid rhinorrhoea: does fibrin glue change the surgical outcome? J Laryngol Otol 2020;134(7):582ŌĆō5.


19) Park JH, Choi JH, Kim YI, Kim SW, Hong YK. Modified graded repair of cerebrospinal fluid leaks in endoscopic endonasal transsphenoidal surgery. J Korean Neurosurg Soc 2015;58(1):36ŌĆō42.



20) Kuan EC, Yoo F, Patel PB, Su BM, Bergsneider M, Wang MB. An algorithm for sellar reconstruction following the endoscopic endonasal approach: a review of 300 consecutive cases. J Neurol Surg B Skull Base 2018;79(2):177ŌĆō83.



21) Yoon TM, Lim SC, Jung S. Utility of sphenoid mucosal flaps in transnasal transsphenoidal surgery. Acta Otolaryngol 2008;128(7):785ŌĆō9.


22) Kim EH, Moon JH, Kim SH. Clipping technique for the repair of the intraoperative cerebrospinal fluid leakage during transsphenoidal pituitary tumor surgery. Oper Neurosurg (Hagerstown) 2019;17(4):382ŌĆō8.


23) Bakhsheshian J, Hwang MS, Friedman M. What is the evidence for postoperative lumbar drains in endoscopic repair of CSF leaks? Laryngoscope 2015;125(10):2245ŌĆō6.

24) Zwagerman NT, Wang EW, Shin SS, Chang YF, Fernandez-Miranda JC, Snyderman CH, et al. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg 2018;131(4):1172ŌĆō8.

-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,550 View
- 90 Download
- Related articles
-
Olfactory Evaluation by UPSIT before and after Endoscopic Sinus Surgery 1996 November;3(2)
Patency of the Artificial Ostium after Endoscopic Sinus Surgery 1994 November;1(2)
The Effect of Smoking on Outcome in Endoscopic Sinus Surgery2009 November;16(2)
Prevention of the Lateral Synechia Formation after Endoscopic Sinus Surgery2010 May;17(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print