|
 |
| J Rhinol > Volume 31(1); 2024 |
|
Abstract
Background and Objectives
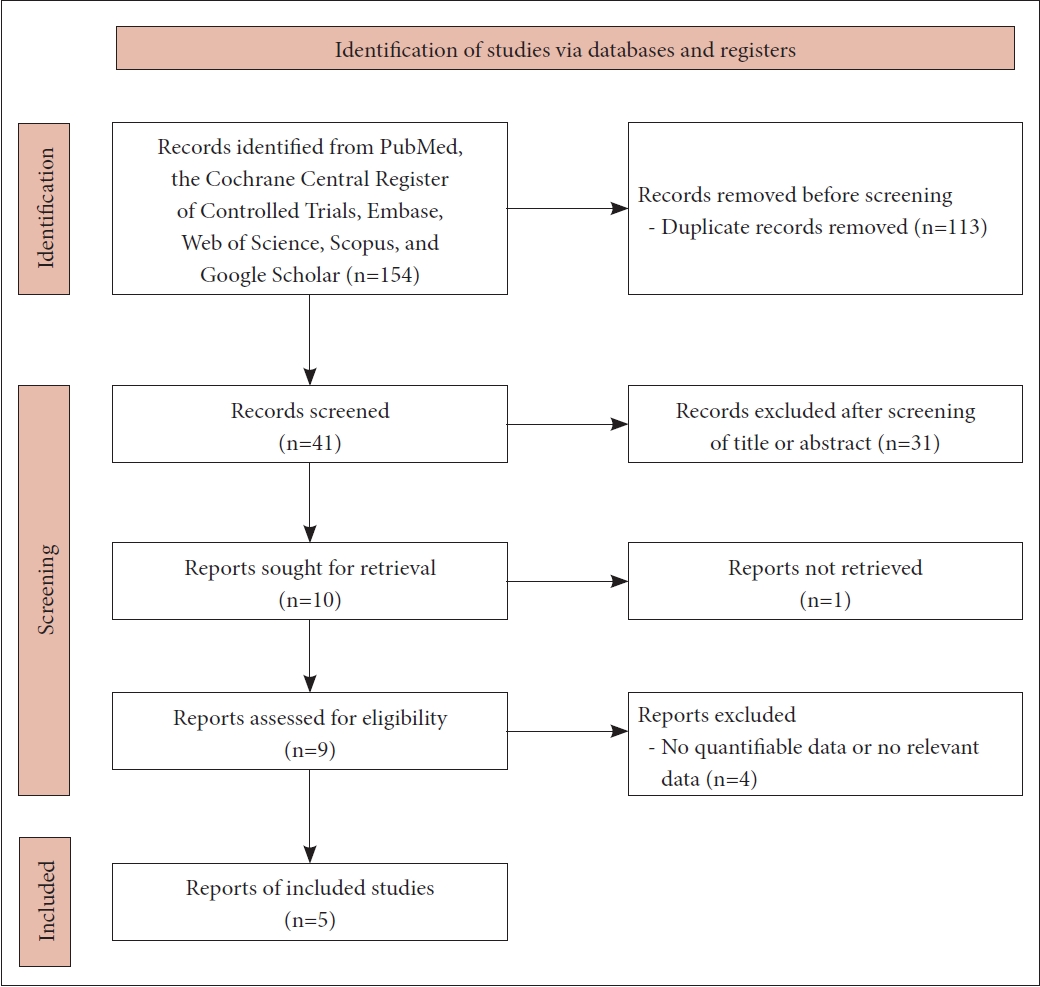
Methods
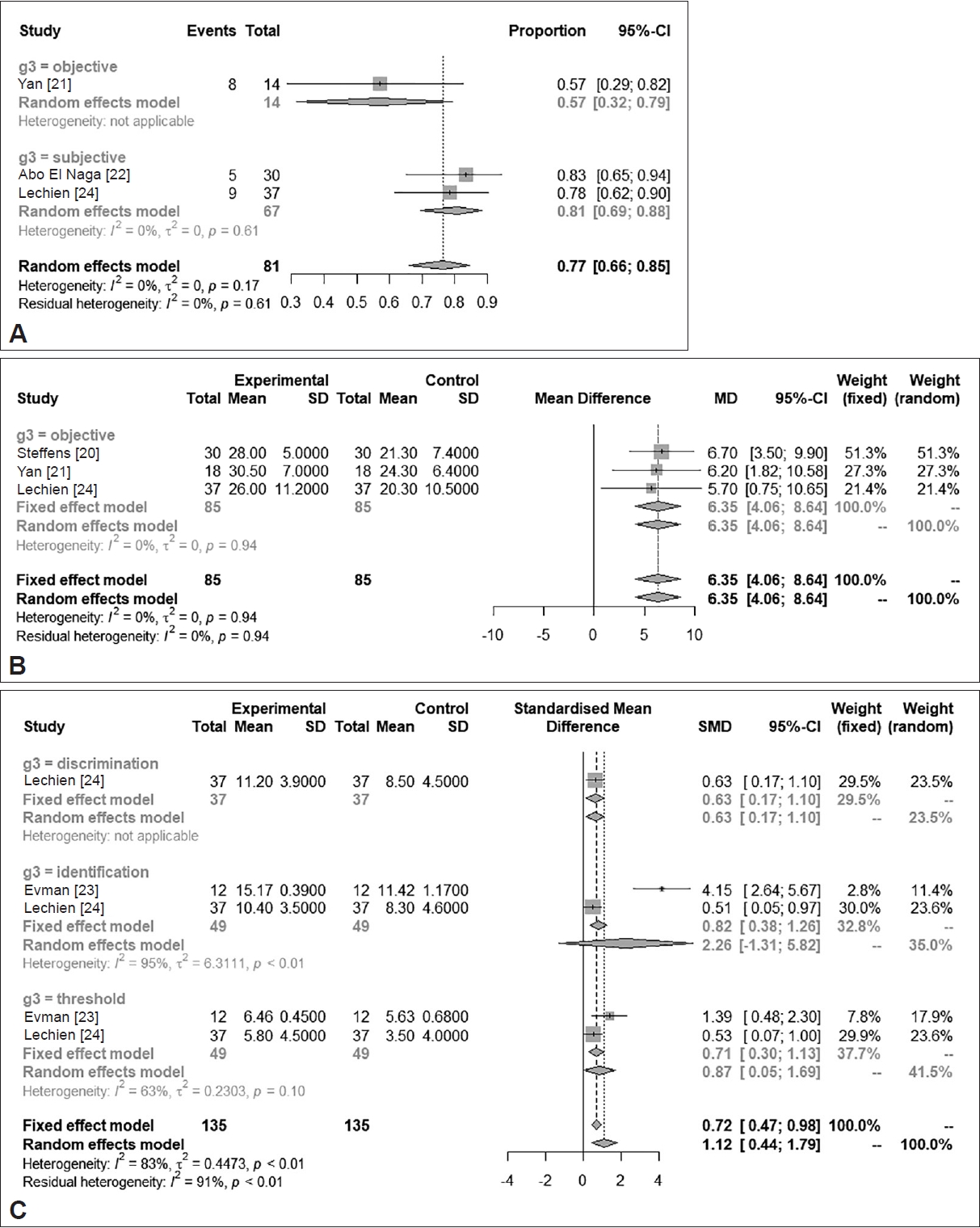
Results
Supplementary Materials
Supplementary Table 1.
Supplementary Table 2.
Notes
Availability of Data and Material
All data generated or analyzed during the study are included in this published article and its supplementary information files.
Conflicts of Interest
Do Hyun Kim and Se Hwan Hwang who are on the editorial board of the Journal of Rhinology were not involved in the editorial evaluation or decision to publish this article. Ah Young Bae has declared no conflicts of interest.
Author Contributions
Conceptualization: Do Hyun Kim, Se Hwan Hwang. Data curation: all authors. Formal analysis: Do Hyun Kim, Se Hwan Hwang. Funding acquisition: Do Hyun Kim, Se Hwan Hwang. Investigation: all authors. Methodology: all authors. Project administration: Do Hyun Kim, Se Hwan Hwang. Resources: all authors. Software: Do Hyun Kim, Se Hwan Hwang. Supervision: Do Hyun Kim, Se Hwan Hwang. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
Fig. 2.
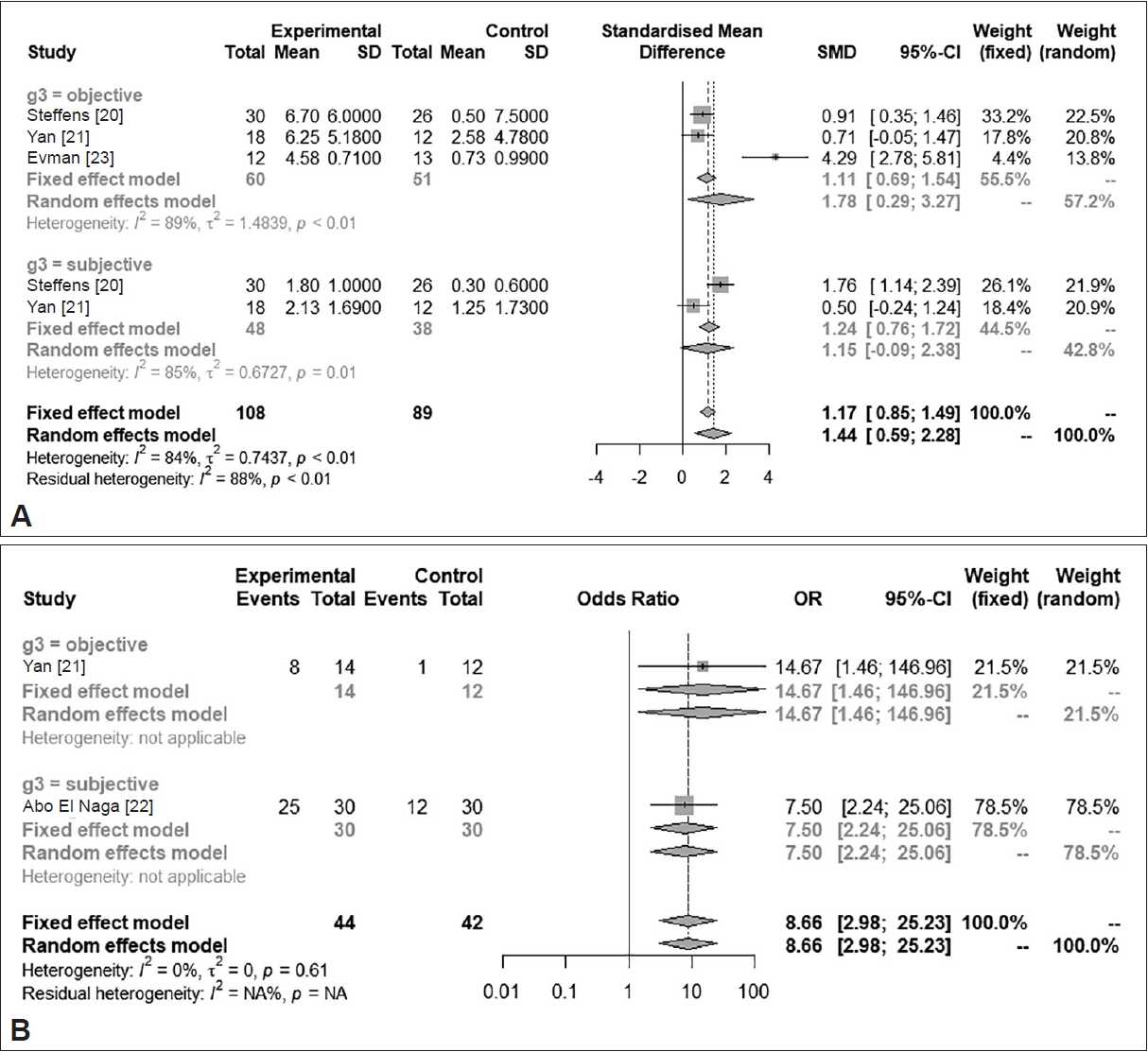
Fig. 3.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement1
Supplement1 Print
Print